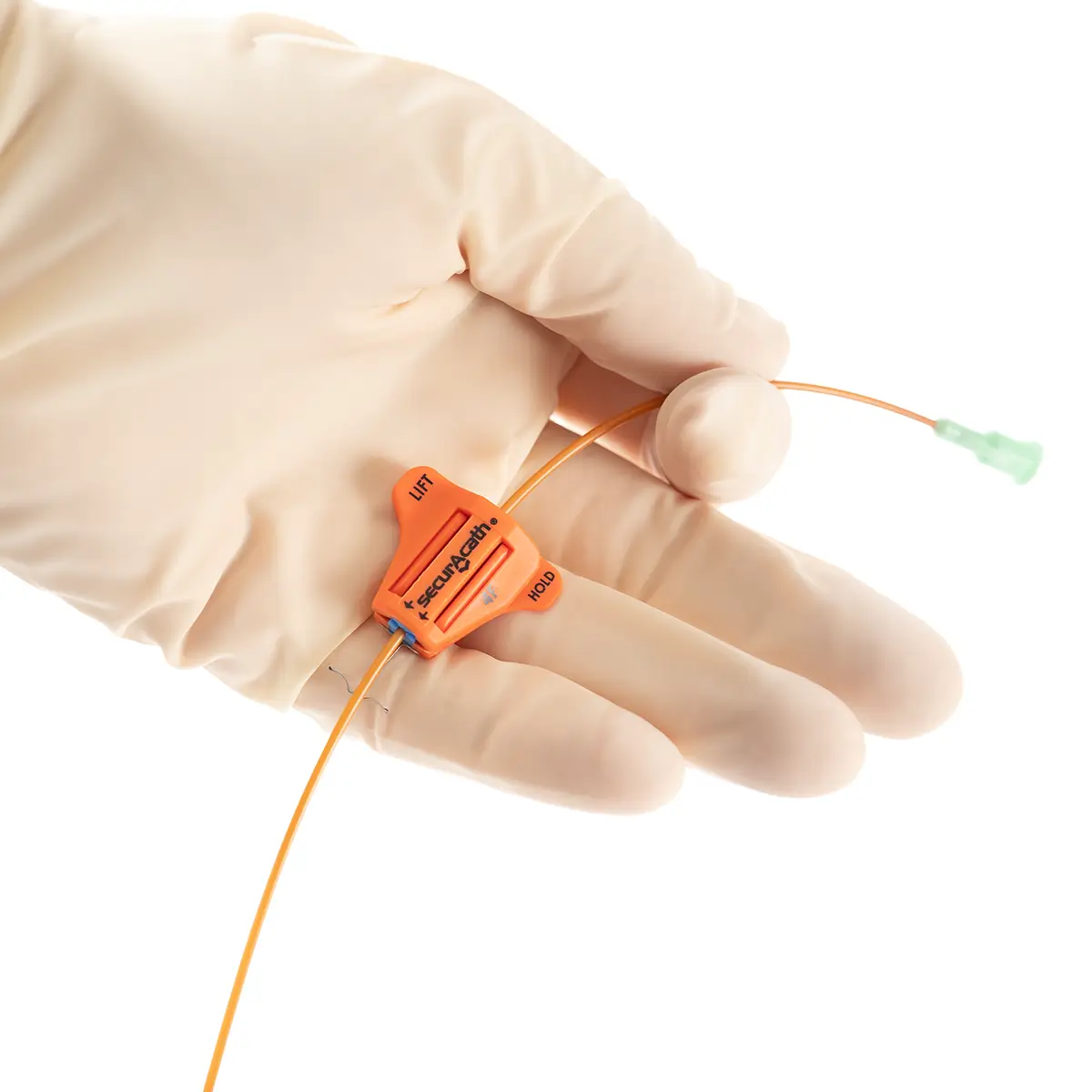
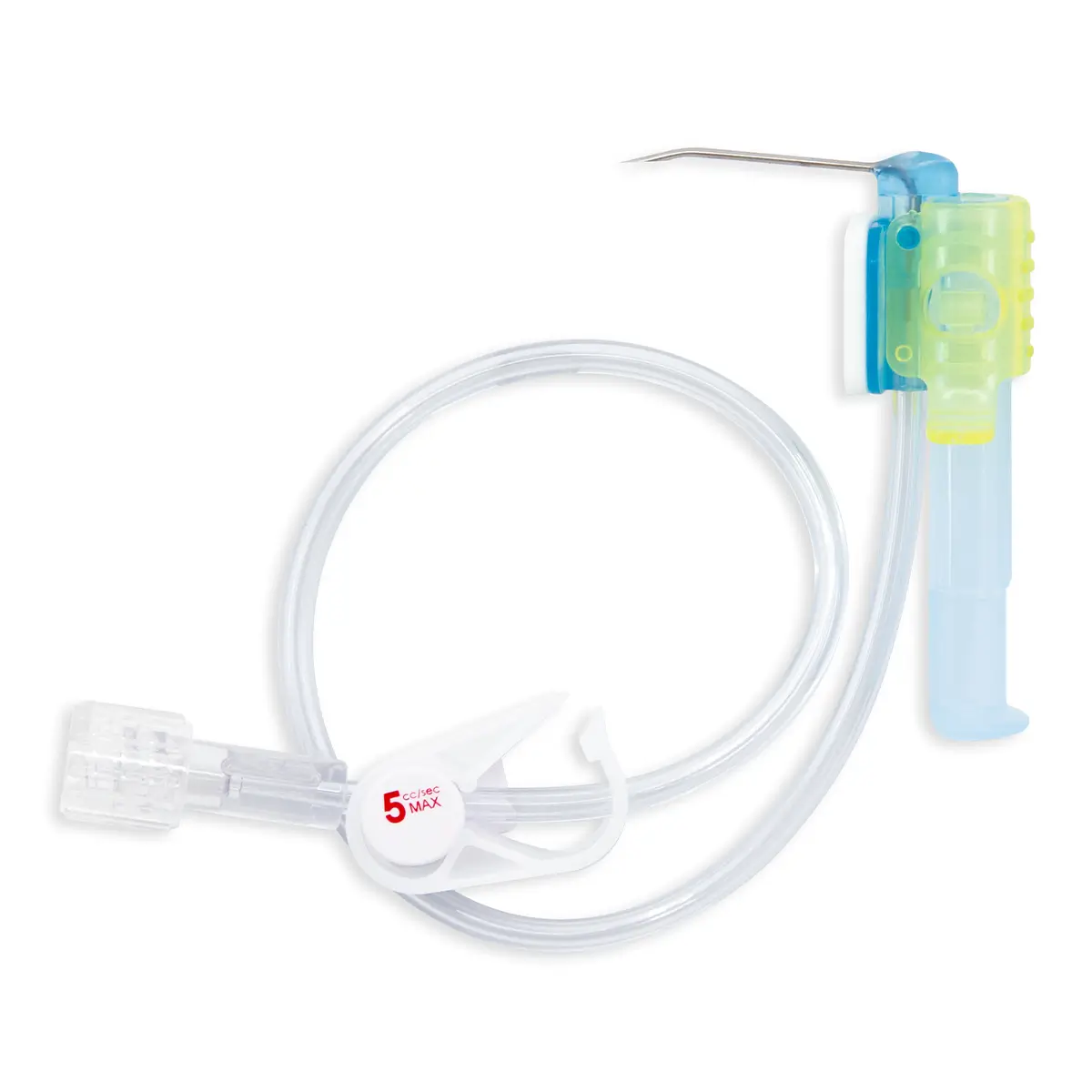
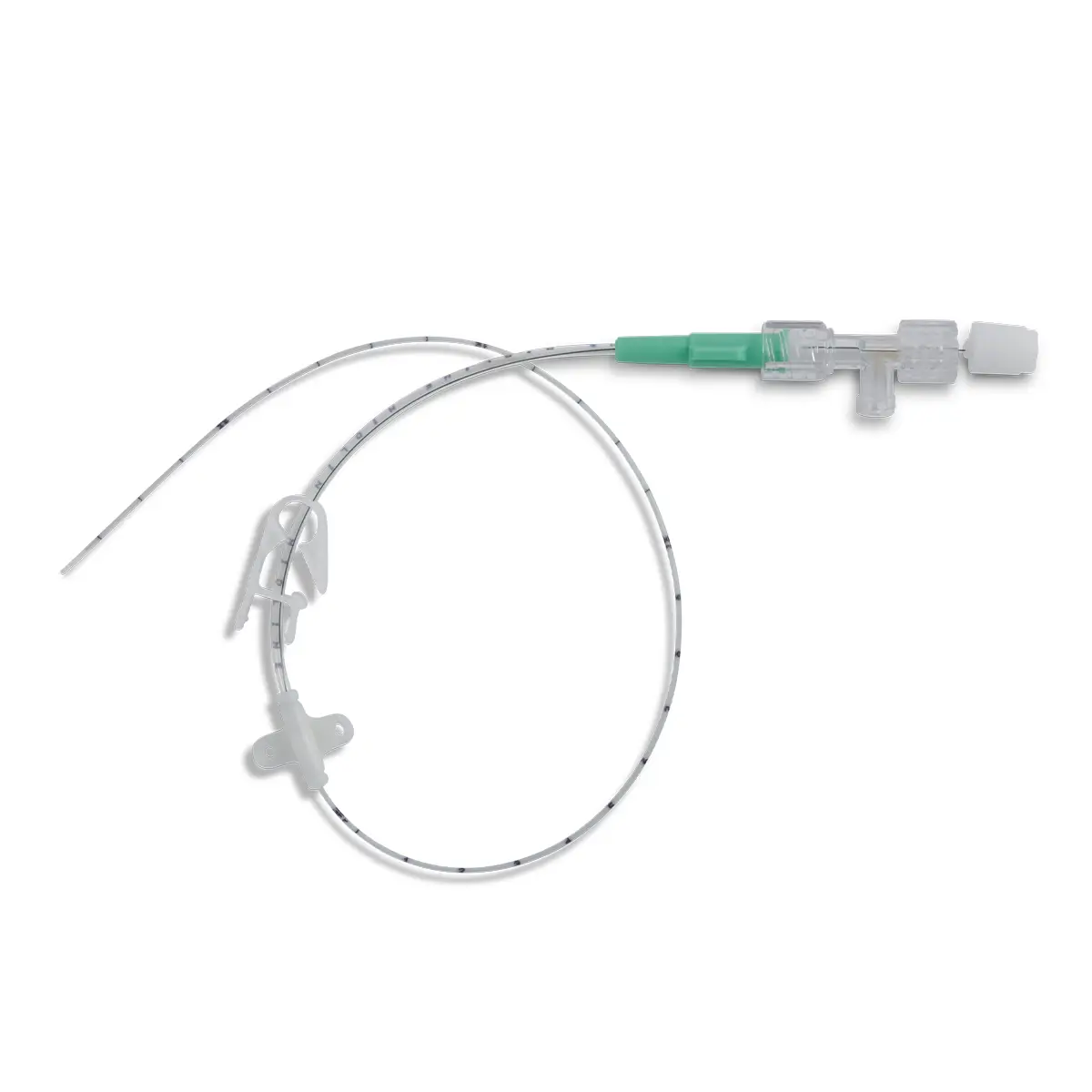
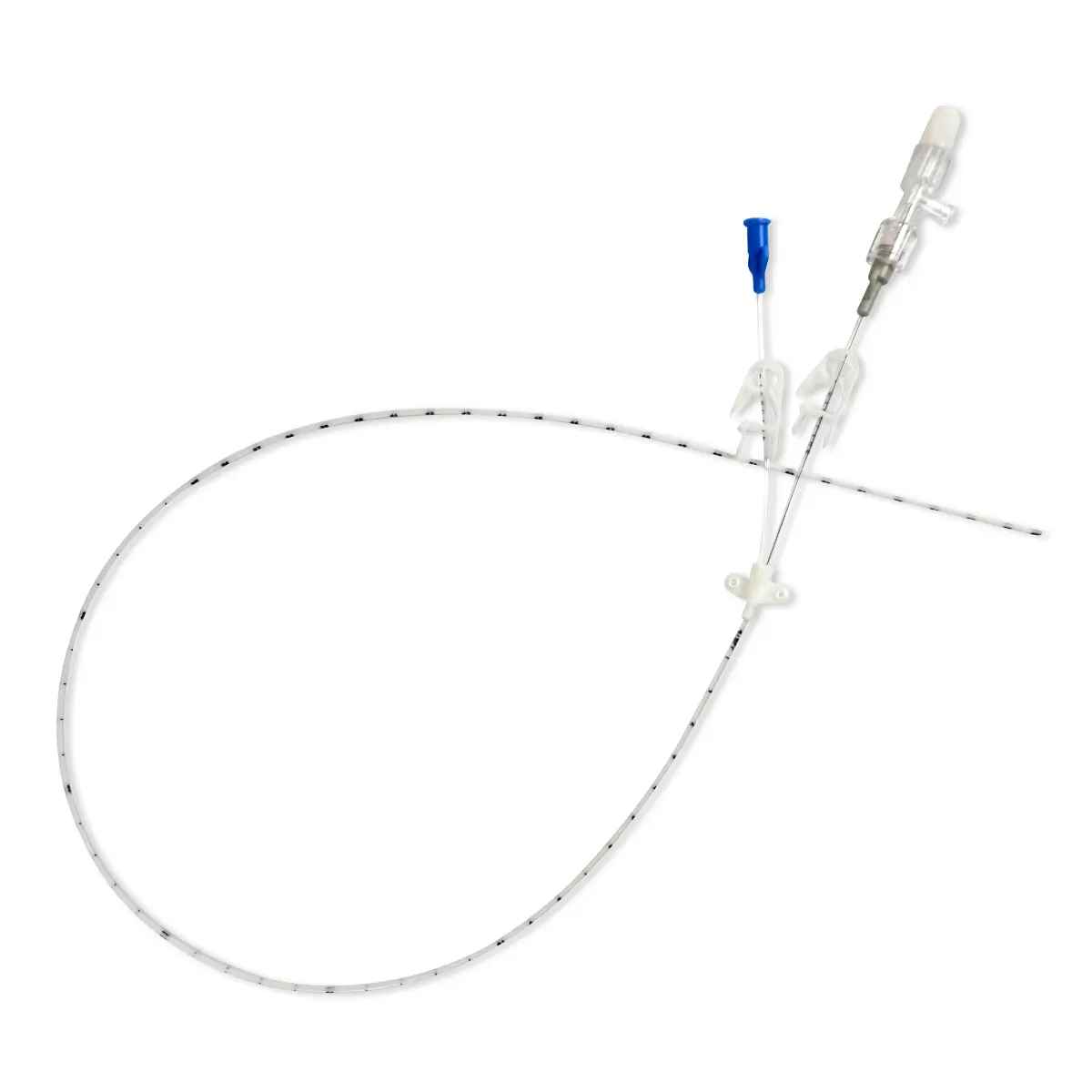
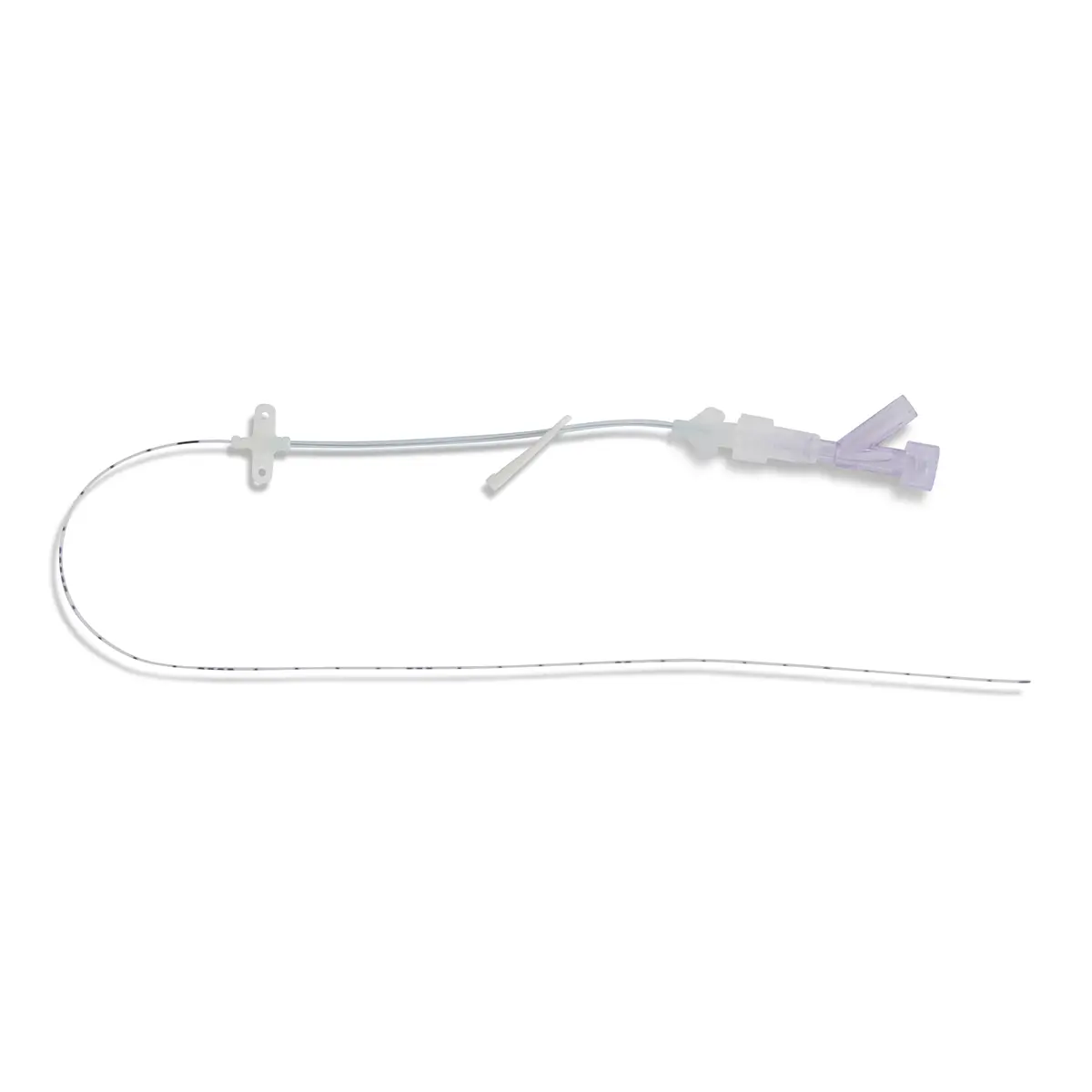
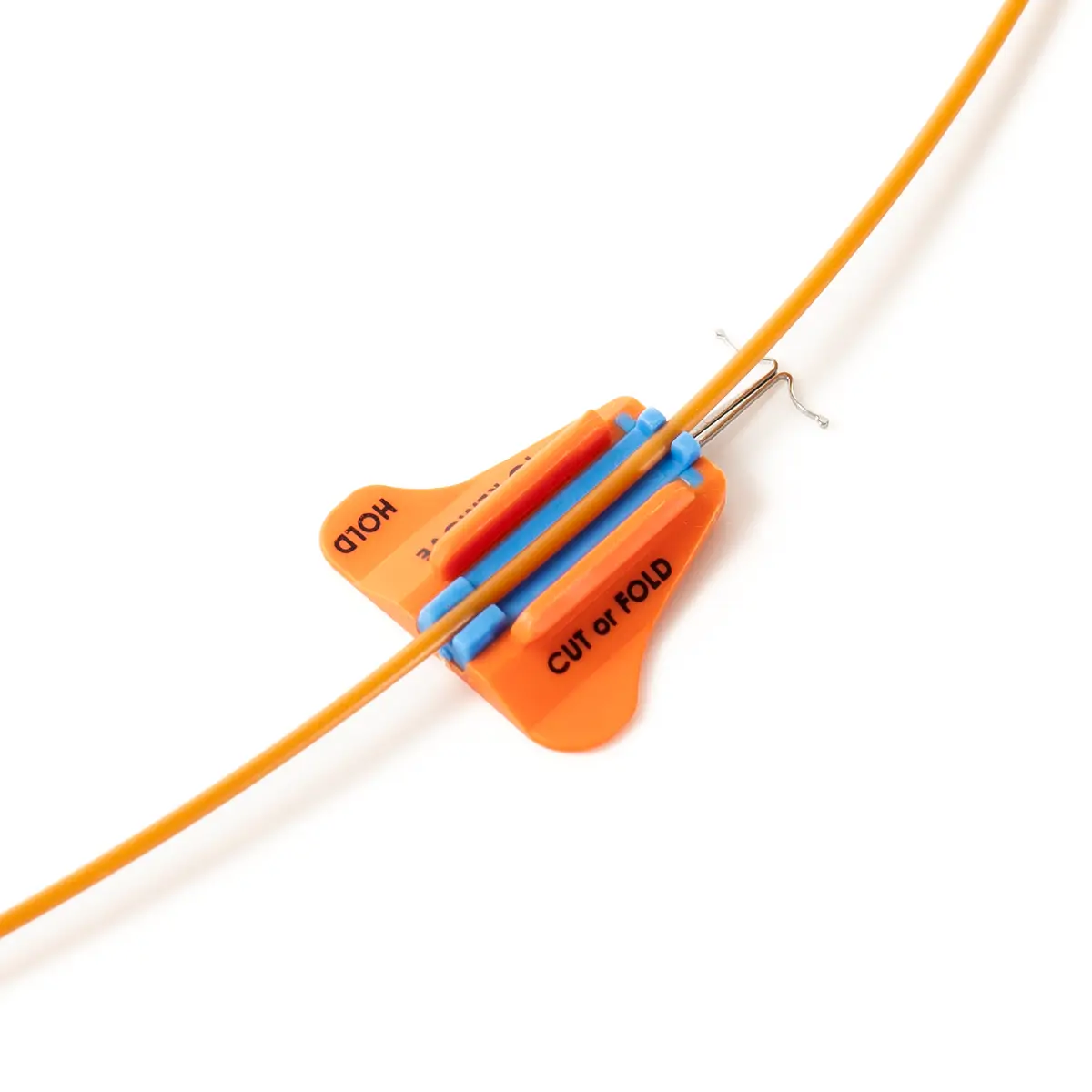
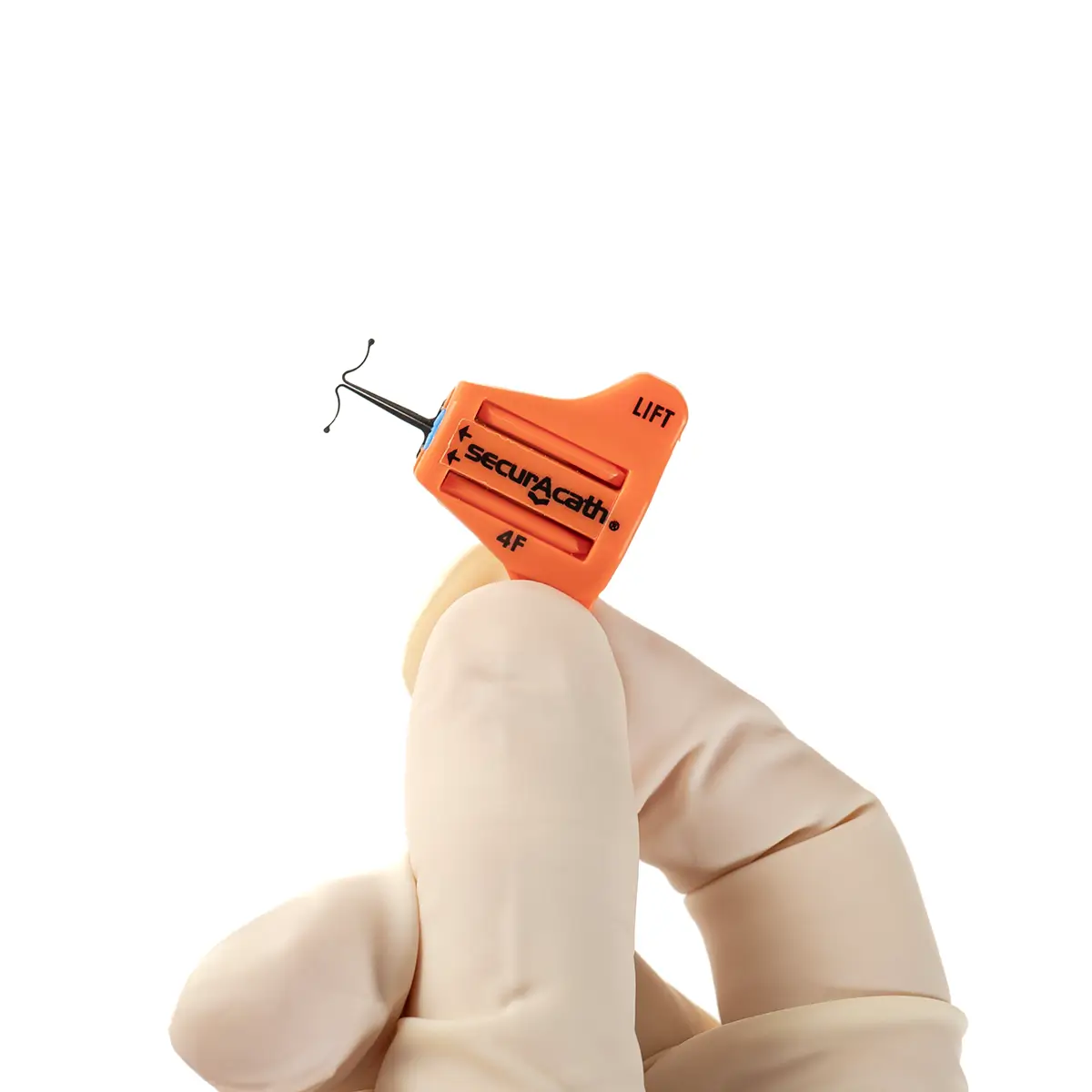
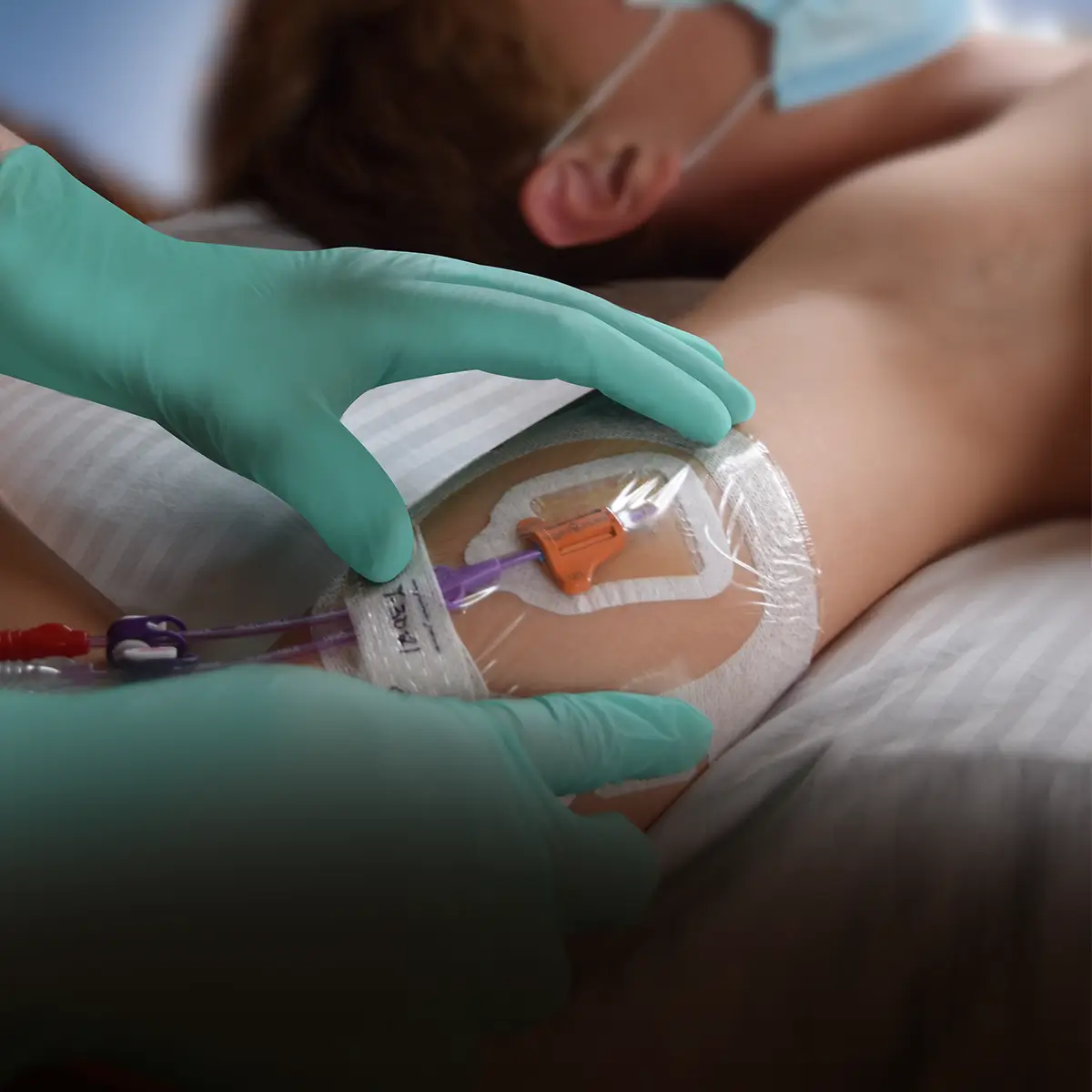
The SecurAcath® Subcutaneous Anchor Securement System (SASS) is a revolutionary new method for catheter securement that does not require sutures or adhesives. The unique design of the SecurAcath® secures right at the insertion site using small, flexible securement feet placed in the subcutaneous tissue just beneath the skin. 6
Used for securing PICC lines, CVCs and other lines for any patients, from neonates to geriatrics.
Product Codes:
| Size |
Code |
| 3F |
VIA400130 |
| 4F |
VIA400140 |
| 5F |
VIA400110 |
| 6F |
VIA400150 |
| 7F |
VIA400120 |
| 8F |
VIA400160 |
| 9F |
VIA400170 |
| 10F |
VIA400180 |
| 12F |
VIA400200 |
References:
[1] McParlan et al, “Intravascular catheter migration: A cross-sectional and health-economic comparison of adhesive and subcutaneous engineered stabilisation devices for intravascular device securement.” Journal of Vascular Access (2019):online
[2] Pittiruti M, et al. “Clinical experience of a subcutaneously anchored sutureless system for securing central venous catheters.” British Journal of Nursing (2019) Jan 24;28(2):S4-14.
[3] Zerla et al. “Evaluating Safety, Efficacy, and Cost-Effectiveness of PICC Securement by Subcutaneously Anchored Stabilization Device.” Journal of Vascular Access 18.3 (2017):238-242.
[4] Dolcino et al. “Potential Role of a Subcutaneously Anchored Securement Device in Preventing Dislodgement of Tunneled-Cuffed Central Venous Devices in Pediatric Patients.” Journal of Vascular Access 18.6 (2017):540-545.
[5] Hughes, Meinir Elen. “Reducing PICC migrations and improving patient outcomes.” British Journal of Nursing 23:Sup1, (2014): S12-S18.
[6] Rowe, et al, “Catheter Securement Impact on PICC-related CLABSI: A University Hospital Perspective” American Journal of Infection Control, Open Access, June 17, 2020
The flexible securement feet are made of Nitinol which is a shape-memory alloy of nickel and titanium. Nitinol is used in several medical devices including self-expanding stents and IVC filters. The plastic is polypropylene and elastomer. The SecurAcath® is not made with natural rubber latex.