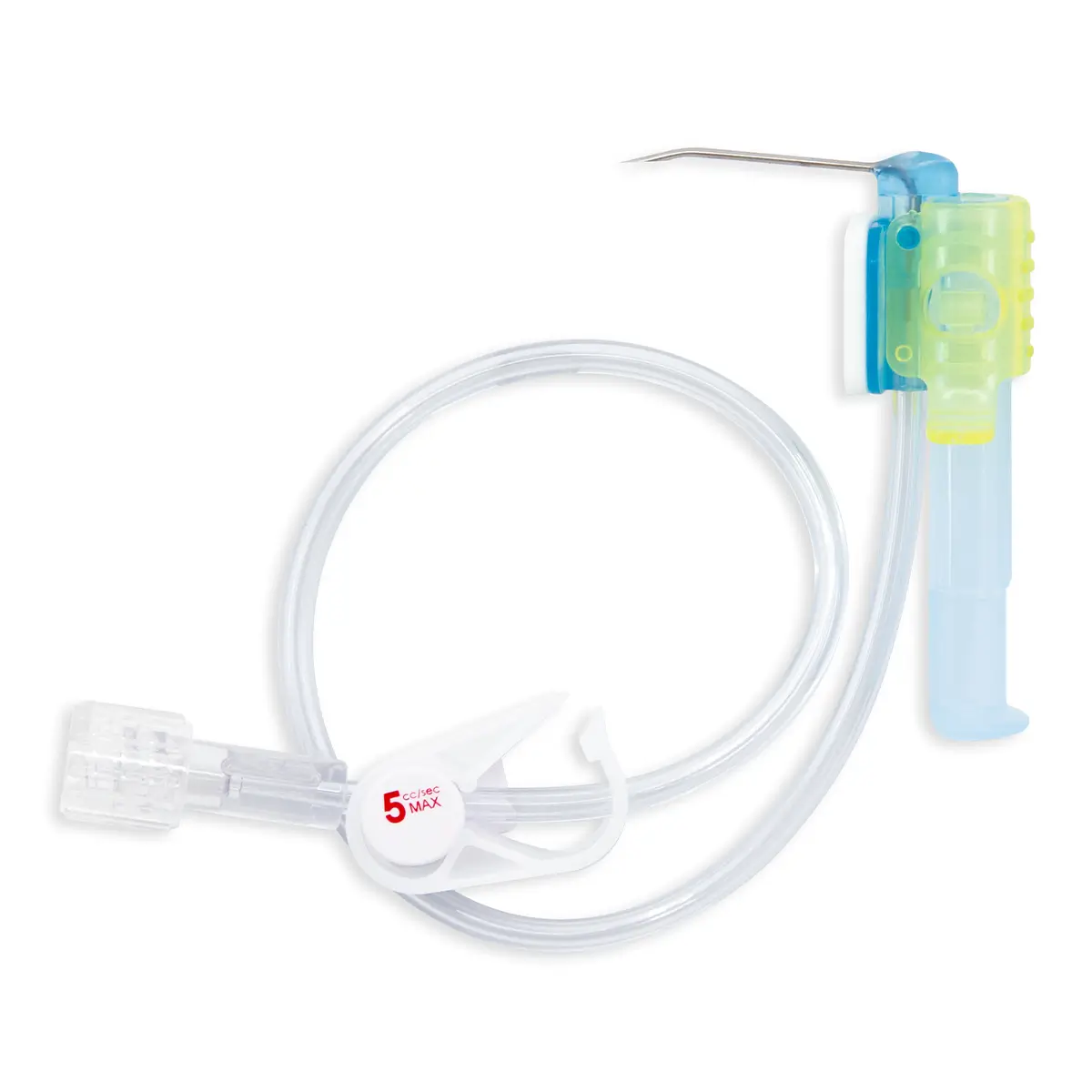
New distributor announced for NICE-approved, NHSE-endorsed catheter securement device, SecurAcath®
Medical technology saving the NHS over £4million each year in England alone
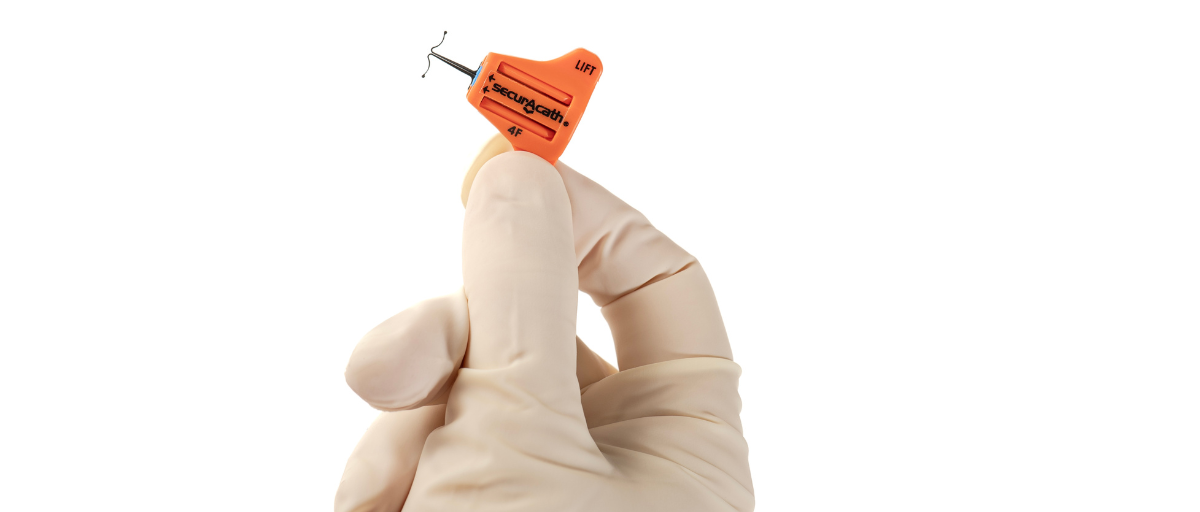
US-based Interrad Medical has partnered with Vygon UK, to distribute its catheter securement device SecurAcath®, across England, Scotland and Wales. With the newest addition to their product range, a 9Fr option, exclusively available through Vygon. This addition to the range enables use on larger multi lumen CVC lines.
Leading medical device company, Vygon UK will provide training on the effective use of SecurAcath®, which is currently being used by more than 100 NHS Trusts nationwide. The company will also further promote the benefits of the device, alongside its complementary vascular access product range.
Endorsed by NHS England (NHSE) as the standard of care for securing peripherally inserted catheter lines (PICC) in the country, SecurAcath® is also recommended by the National Institute for Health and Care Excellence (NICE). It describes the product as ‘a cost-saving option for securing peripherally inserted central catheters (PICCs) with an anticipated medium- to long-term dwell time.’
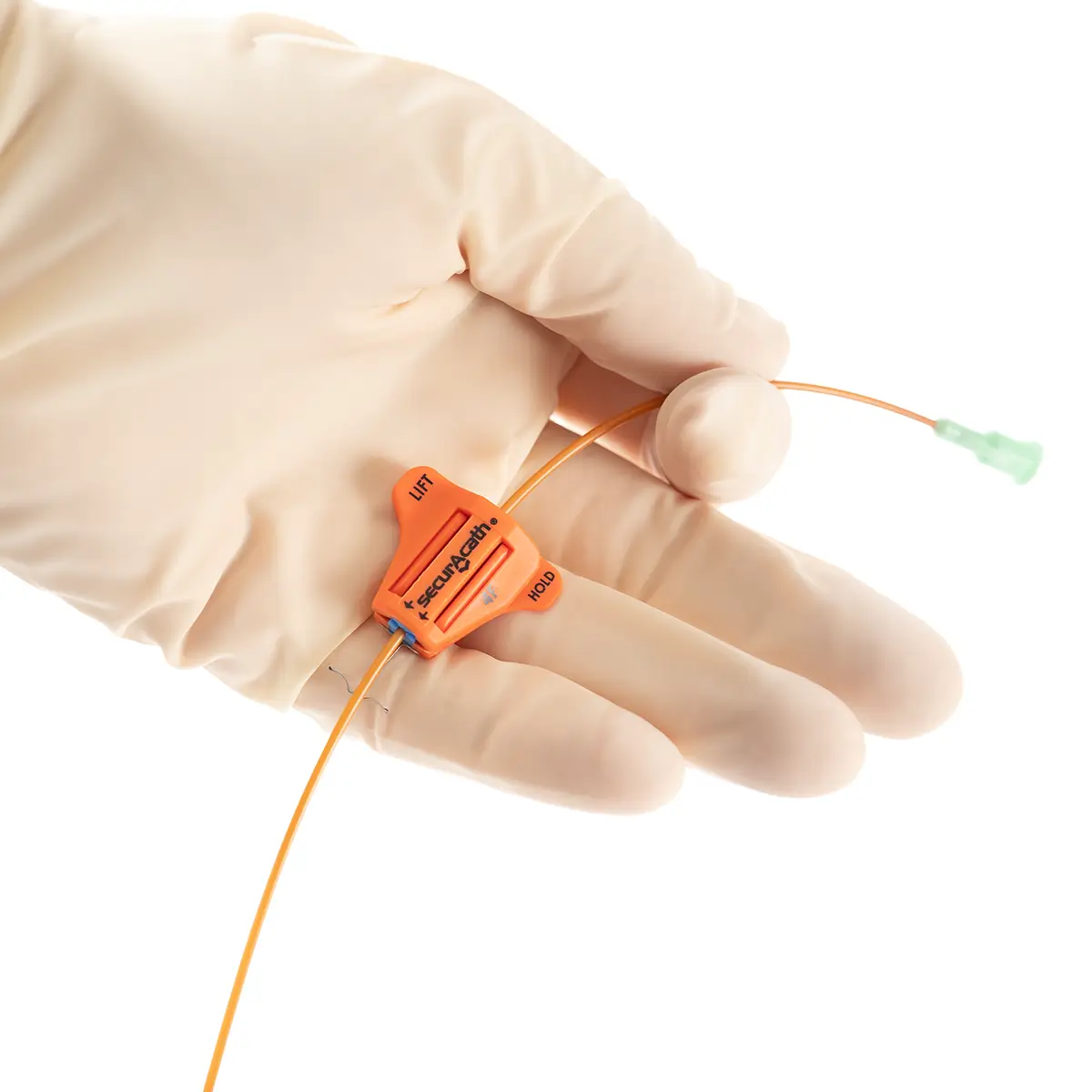
First introduced to the UK in 2011 following clinical demand, the single-use device secures percutaneous catheters in position on the skin and can be used on both adults and children. Reducing catheter complications and cost savings, cost modelling indicates reductions of between £9-£95 per patient and annual savings of more than £4.2million to the NHS in England.
The efficacy of SecurAcath® has been proven in over 25 medical studies and positively reinforced in peer-reviewed publications. A 2023 study of ‘subcutaneous anchor securement systems in oncology patients’[1] reviewed ten years of SecurAcath® use at the Clatterbridge Cancer Centre in Liverpool.
With over 9,200 patients and more than a million catheter days, the retrospective study demonstrated SecurAcath®’s superiority in assisting the patient to reach the end of need with a single PICC.
It compared the results of an Adhesive Securement Device (ASD) to SecurAcath®- a Subcutaneous Anchor Securement System (SASS) and found that partial or complete dislodgement causing the unplanned removal of the PICC occurred at 12% for ASD and only 0.4% for SASS.
Additionally, it found a 68% probability of reaching the end of need with one PICC at two years for patients with an ASD, regardless of the reason for premature removal. Whereas, for patients with a SASS, it was over 95%.
As one of only four technologies to be awarded Innovative Technology Payment (ITP) programme status[2], SecurAcath® was provided to NHS Trusts free of charge from October 2018 until March 2021 – helping to grow its use within the health service from 20-80% over the past five years.
SecurAcath® offers a natural synergy for Vygon UK, joining its award-winning vascular access product portfolio, which features everyday consumables vital for the smooth running of the NHS, such as PICCs, midlines, ports, catheters and much more.
Education and guidance on how to effectively use the device will also be provided through Vygon’s Clinical Nurse Advisors (CNAs), clinical support packs, online resources, and study days.
James Leek, Business Unit Manager at Vygon, explains: “At Vygon we pride ourselves on providing the NHS with the best tools – medical devices that are at the cutting edge of technology, that are helping to lighten the load, improve efficiencies and reduce costs. SecurAcath® does all these things and more. So, we can’t wait to get out to the market and start introducing its proven benefits to more of our customers.”
Jeff Killion, Executive Vice President of Interrad Medical, said: “We are excited to partner with Vygon UK for distribution of the SecurAcath®. Vygon has had great success with the SecurAcath® in several other countries and we look forward to working with the UK team to continue growth in England, Scotland and Wales.”
Vygon Group, which has subsidiaries across Europe, the Americas, and Asia Pacific is already the distributor of SecurAcath® in France, Spain, Poland, Switzerland, Belgium, The Netherlands and Luxembourg.
Designed to secure the catheter for the entire duration of therapy, eliminate catheter movement dislodgement, and prevent therapy interruption, SecurAcath® features small, blunt, flexible securement feet which are placed just beneath the skin to stabilise the catheter right at the insertion site. For more information, visit https://securacath.com/
Notes to editors
For further information and imagery please email vygon@thetoniccomms.co.uk
About Interrad Medical, Inc.
Plymouth, Minnesota-based Interrad Medical, Inc. is the developer and manufacturer of the SecurAcath® Subcutaneous Anchor Securement System (SASS), a revolutionary new method for catheter securement that does not require sutures or adhesives. The SecurAcath® is the only subcutaneous catheter securement device in the world. SecurAcath® has gained more positive clinical evidence and guidance support than any other catheter securement device.
About Vygon
We are a leading supplier of medical and surgical devices dedicated to helping clinicians deliver the best possible outcomes for their patients. Our reputation for delivering high quality products is supported by our resolve to provide excellent customer service at whatever level is required. Our comprehensive training and education programme is committed to building clinical competencies, knowledge, and expertise whilst Vygon’s skilled Technical Team focuses on providing workable solutions to a wide range of practical queries. We take particular pride in our partnerships with hospitals and Trusts all over the UK where we work closely with both healthcare and procurement professionals to combine clinical effectiveness and financial efficiency.
References
[1] A retrospective study of subcutaneous anchor securement systems in oncology patients by Hawes, M. McCormick, C. Gilbert, G., Journal of Vascular Access, 2023’
[2] The ITP concluded in March 2021 and evolved into the Medtech Funding Mandate programme: https://www.england.nhs.uk/aac/wp-content/uploads/sites/50/2021/01/mtfm-policy-guidance-jan-2021.pdf