21 Hours Saved in Critical Care Patient Recovery
Discover how the team from Norfolk and Norwich University Hospital improved patient outcome and saved 21 hours in stay length for post Ovarian Cytoreductive Surgery (CRS) patients, using goal-directed intra-operative fluid management (GDFM) guided by the MostcareUp cardiac output monitor.
Case Study Overview:
With a critical care or ITU patient every second counts when it comes to life-saving interventions and improving patient outcomes. This is especially prevalent after extensive surgery such as ovarian cytoreductive surgery (CRS). Post-CRS patients may present with high fluid shifts, low albumin, and different intra-abdominal pathology which can add additional complications to their recovery.
The team at Norfolk and Norwich University Hospital identified an opportunity to improve patient outcomes following extensive abdominal surgery by using goal directed intra-operative fluid management, guided by a cardiac output monitor.
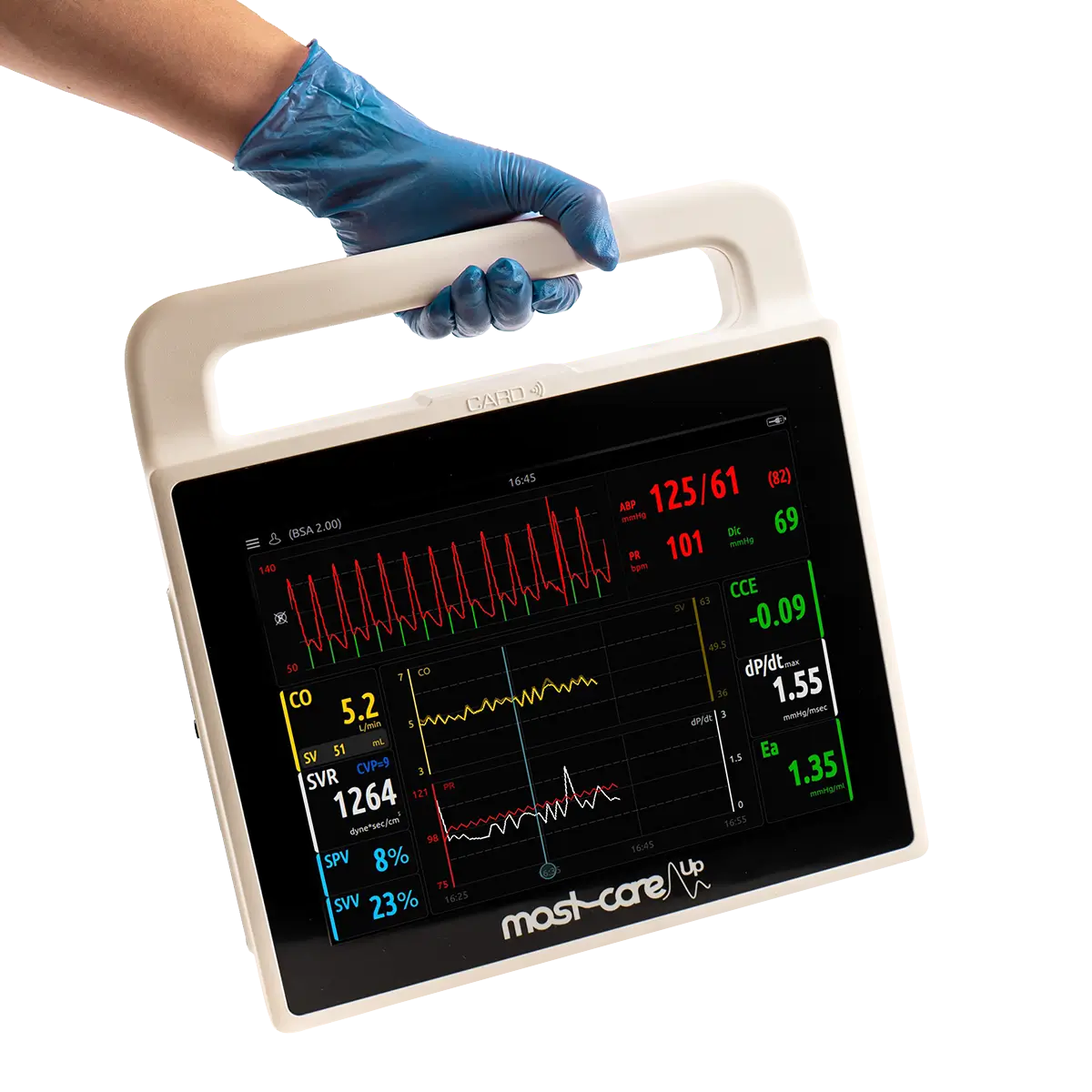
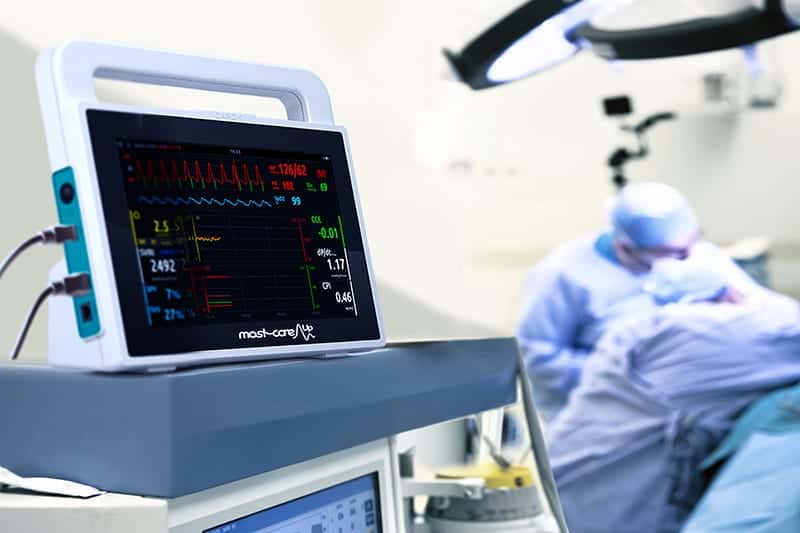
For the purpose of this study, Norfolk and Norwich University Hospital introduced the MostcareUp Haemodynamic Monitor to the critical care department.
Read below to discover the highlights from their findings or follow the button to request the full study.
Patient Statistics prior to procedural improvement:
For the team at Norfolk and Norwich University Hospital, previous patients presenting to a tertiary centre critical care unity post-operatively after CRS showed:
- Median age 72 years, 78% had Group 3 disease (inc. upper abdominal procedures)
- Mean length of critical care stay was 47 hours (IQR 26-67)
- Median duration of vasopressor support was 36.5 hours (IQR 27-54)
- 10% of patients suffered acute renal injury or failure, a further 14% determined ‘at risk’
- 10% of patients suffered from respiratory complications, including document atelectasis and pleural effusions with requirements from both non-invasive and invasive ventilator support.
- Median fluid balance in the first 24 hours 1583ml (IQR 1988-3602ml), with median fluid input in the first 24 hours of 4410ml (IQR 3603-56643ml)
- Cardiovascular support: 32% required no CVS support, 50% required Noradrenaline, 18% required Metaraminol
To combat these issues, the team at Norfolk and Norwich University Hospital introduced goal-directed intra-operative fluid management (GDFM) guided by the MostcareUp cardiac output monitor, to identify a clear case for improved patient outcome.
Study Results
21 hour Reduction
In critical care stay time for patients within the study
100% Reduction
In additional respiratory or renal complications
10% Increase
In Patients Requiring no CVS Support post surgery
To see the full results from this case study and impacts on patient outcomes, request the full case study from marketing@vygon.co.uk
Conclusion
Although numbers in this study are small, there is a clear suggestion in improvement of patient outcomes and use of finite resources within this department, which stem from the use of intra-operative goal-directed fluid management assisted with a cardiac output monitor.
Positive indications from this study suggest that there was a decreased requirement and usage time of vasopressors, and the numbers of patients developing post-operative complications reduced. Due to the reduction in complications, this study also ultimately demonstrates a decrease in length of critical care stay.
Method
The team at Norfolk and Norwich University Hospital are certified by the European Society of Gynaecology Oncology.
This study was conducted over a 5-month period, with 10 patients undergoing CRS for advanced ovarian cancer had their surgery with a cardiac output monitor during their intra-operative management (keeping the SVV <10) and for 24 hours post-operatively. Data was collected retrospectively from all 10 cases of these 10, 5 received post-operative care in the critical care unit.
To find out more about the implementation and roll out of goal directed fluid management and cardiac output monitoring, or to request the full study please email marketing@vygon.co.uk